Targeted protein degraders (TPDs), also known as proteolysis targeting chimeras (PROTACs), are a compelling modality within the pharmaceutical industry, used in drug design for precise protein degradation through chemical biology. This innovative drug-design approach is particularly effective against targets traditionally deemed “undruggable.” TPDs, which are bifunctional compounds connected by a linker, leverage the ubiquitin-proteasome system to initiate a series of enzymatic steps, resulting in the targeted destruction of specific proteins.
One component of these compounds acts as a ligand binding to the ubiquitin-protein ligase (E3), while another serves as a ligand for the target protein. This strategic pairing brings the target protein into proximity with the E3 ligase, forming a complex that transfers ubiquitin tags to the target protein. This, in turn, leads to the recognition and therapeutic knockdown of the protein by the proteasome. Notably, for targets with slow turnover, the pharmacological effect can persist well beyond the degrader’s pharmacokinetic half-life.
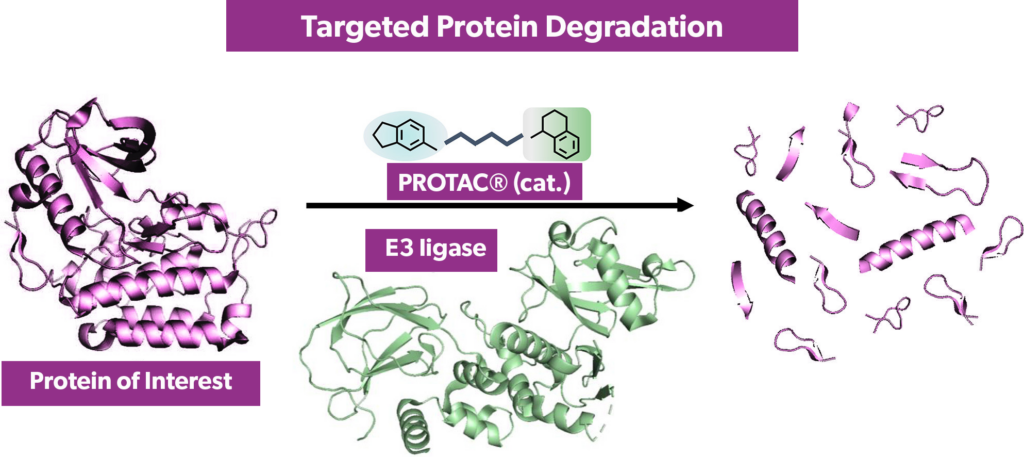
Introduced in the early 2000s, TPDs entered clinical trials in 2019 with the oral administration of bavdegalutamide. Despite their promise in drug development, these bifunctional compounds pose unique challenges. Chemically, they surpass Lipinski’s Rule of 5, typically featuring molecular weights between 600 and 1200 mass units, high polar surface areas, and a high number of rotatable bonds. These characteristics lead to unfavorable physicochemistry, resulting in challenges such as low solubility; issues with non-specific binding; poor permeability; and, subsequently, poor oral bioavailability.
The complexity of optimizing TPDs contributes to a slower drug discovery process compared with small molecules. However, more than ten oral TPDs are currently in phase I and II clinical trials, with bavdegalutamide entering phase III, underscoring the promising role of TPDs in drug development despite these hurdles.
Understanding the importance of addressing these challenges, Admescope has actively engaged in transforming ADME assays into a format suitable for protein degraders. This initiative aims to enhance the usability and predictability of related data. While Admescope’s focus is on ADME, Symeres has generated a tool-box platform for PROTACs, and other new bifunctional modalities to support our clients’ needs.
Delving into more nuanced modifications, incorporating buffer modifiers stands out as a typical practice to enhance solubility and reduce non-specific binding, particularly in permeability assays. However, this necessitates a cautious interpretation of results. Notably, cell-based transwell assays (Caco-2 / MDCK) typically perform better than PAMPA, which exhibits a poor correlation for this class of compounds.
Solubility assays are often conducted in biorelevant media (FaSSIF, FeSSIF) and with logD evaluation relying on chromatographic retention time, surpassing the traditional shake-flask approach for these compounds. In the assessment of plasma protein binding, employing several plasma dilutions alongside protease/esterase inhibitors for compound stabilization proves advantageous. Extended experiment times (e.g., 24 hours) in equilibrium dialysis methods enhance data reliability. Alternatively, pre-treated ultrafiltration tubes or TRANSIL-assays offer solutions for compounds prone to plasma instability or non-specific binding.
For metabolism studies, prolonging incubation times to augment metabolic turnover is advisable, while being mindful of the specific limitations of each system (e.g., hepatocytes or liver microsomes). Recognizing potential non-CYP metabolic routes, the use of hepatocytes or the liver S9 fraction is recommended over liver microsomes. At the technical level, incubating each time point individually and introducing the quenching solvent directly into the incubation well, as opposed to collecting multiple samples from the same well, proves advantageous in mitigating the impact of challenging physicochemical properties.
With keen interest, we continue to monitor the evolution of cutting-edge drug modalities, employing a highly adaptable experimental approach to ensure exceptional quality across diverse drug discovery programs. If you want to discuss how we could support your project, feel free to reach out to speak to our experts!

