Time is of the essence when progressing hits to leads and on to preclinical candidates of drug discovery & development. Swift access to ADME-Tox / in vivo PK data is indispensable for expediting the design-make-test cycle.
We support also projects in need for time-critical early ADME characterization. Our expertise lies in swiftly providing essential information tailored to your project’s needs, with the right assays conducted at the right time. Whether as part of an integrated drug discovery program with Symeres or as a standalone service within the Admescope platform, we’re dedicated to bringing efficiencies to your drug discovery journey.
As a standalone early in vitro ADME screening, we have automatized assays ensuring obtaining fast and accurate data for solubility, logD, metabolic stability, PPB and CYP inhibition. For frequently occurring screenings, the data is available within a week!
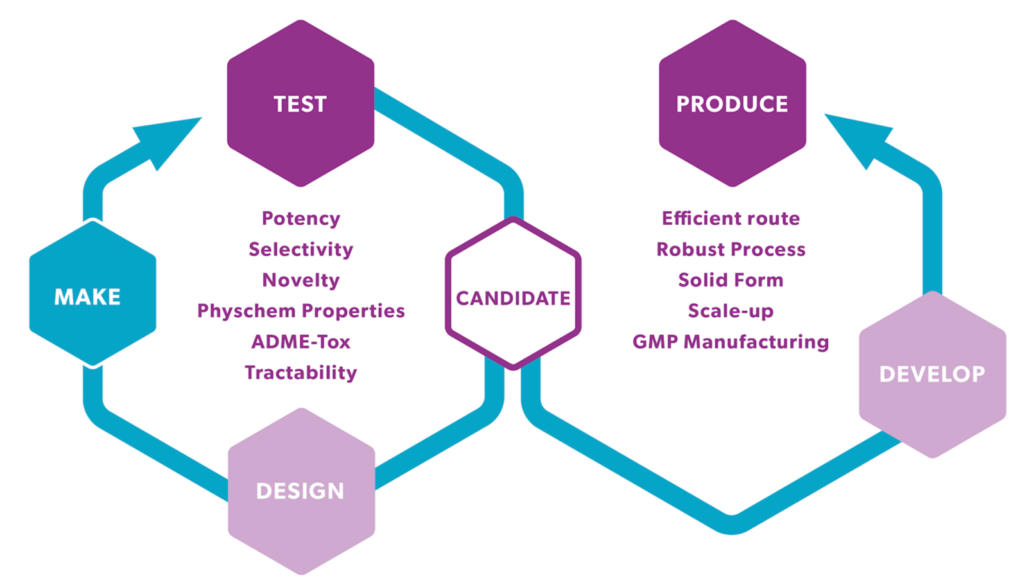
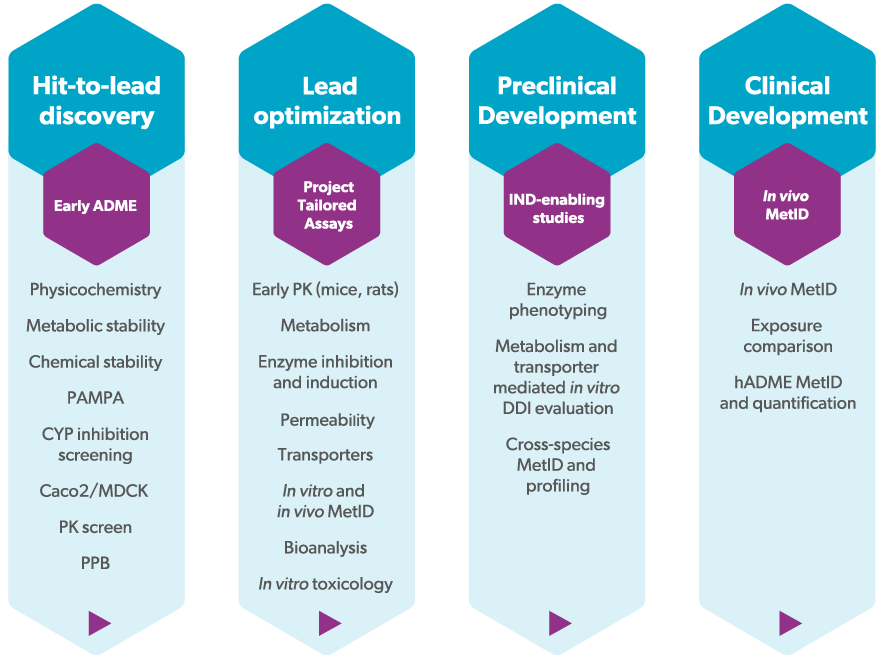
As part of integrated drug discovery projects at Symeres, our suite of early in vitro ADME assays offers comprehensive insights crucial for the medicinal chemistry teams. These include tests governing physicochemical characteristics, metabolic and chemical stability evaluations, permeability assessments (such as PAMPA, Caco2, or MDCK-MDR1 screening), along with plasma protein binding studies, coupled with preliminary in vivo pharmacokinetic screening in our AAALAC accredited animal unit.
Beyond early ADME, our extensive portfolio extends to cover the entire spectrum of non-clinical in vitro ADME-Tox and in vivo DMPK assays. Regardless of your project’s stage, our capabilities stand ready to provide dedicated support covering all drug modalities.
Get in touch with our team to discuss more into our capabilities or to request a quote.
