Our services for Safety Metabolism research include
- Metabolite profiling and characterization in pre-clinical species (in vitro and in vivo MetID)
- Human metabolite profiling and characterization (FIM, SAD, MAD)
- Human MIST analysis (MAD + preclinical safety species)
- Metabolite profiling and characterization in radiolabeled human ADME study
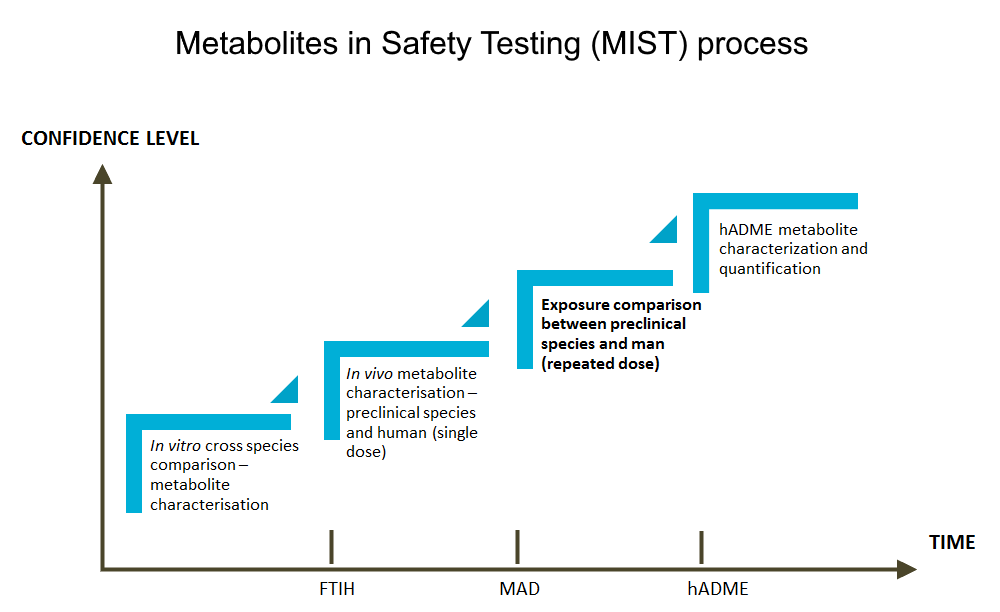
After administration, a drug is usually converted to a variety of metabolites in the liver, GI-tract and/or extrahepatic tissues. Due to species-, strain- and sometimes sex-specific isoforms and metabolic capacities of enzymes, not only the parent drug but also its metabolites that should be tested for its pharmacological and toxicological effects during pre-clinical safety testing of drug candidates. For safety purposes, it is especially crucial to identify metabolites that are disproportionate or unique in humans.
Our services help you to confirm the exposure to human metabolites is adequate in preclinical safety species, or to effectively identify potential MIST issues in time through a battery of different types of studies. While the first investigations related to the topic are performed as in vitro cross-species metabolite profiling and identification, the workflow is continued with various in vivo metabolite characterization studies, using samples from animal and human studies with cold and radiolabeled study compounds.
Exploratory in vivo studies are performed with non-labeled compounds and LC/MS analysis of plasma samples from pre-clinical toxicological studies and first in man studies, to understand the probability of a MIST issue, while steady state exposure comparison between preclinical species and human is the ultimate study to evaluate if human metabolites are toxicologically qualified. Metabolite profiling and identification from human radiolabeled ADME study is the optimal way to define which human metabolites fall under the criteria of FDA and ICH guidelines.
Disproportionate metabolite – when a metabolite is produced at significantly higher extent in humans than in preclinical safety species.
Unique human metabolite – when a metabolite is exclusively produced in humans.
MIST – Metabolites in Safety Testing. The FDA guidance (CDER. Guidance for Industry: Safety Testing of Drug Metabolites) provides recommendations on when and how to identify and characterize drug metabolites whose nonclinical toxicity needs to be evaluated, while further harmonization of the nonclinical safety studies of clinical development among the regions of European Union, Japan, and the United States can be found from the ICH M3 guidance (M3(R2):Guidance on nonclinical safety studies for the conduct of human clinical trials and marketing authorization for pharmaceuticals).
