We are happy to continue our internal R&D news with a new service launch!
The Biopharmaceutics Classification System (BCS)-based biowaiver approach provides an alternative to clinical bioequivalence studies by establishing bioequivalence through in vitro methods. Bioequivalence is confirmed when two drug products exhibit similar absorption rates and extents. BCS-based waivers apply to oral dosage forms classified as BCS Class I (high solubility, high permeability) or Class III (high solubility, low permeability). Permeability assessments, conducted using Caco-2 cells, mimic the human gastrointestinal tract. For a compound to qualify as highly permeable, absorption must occur exclusively via passive transport.

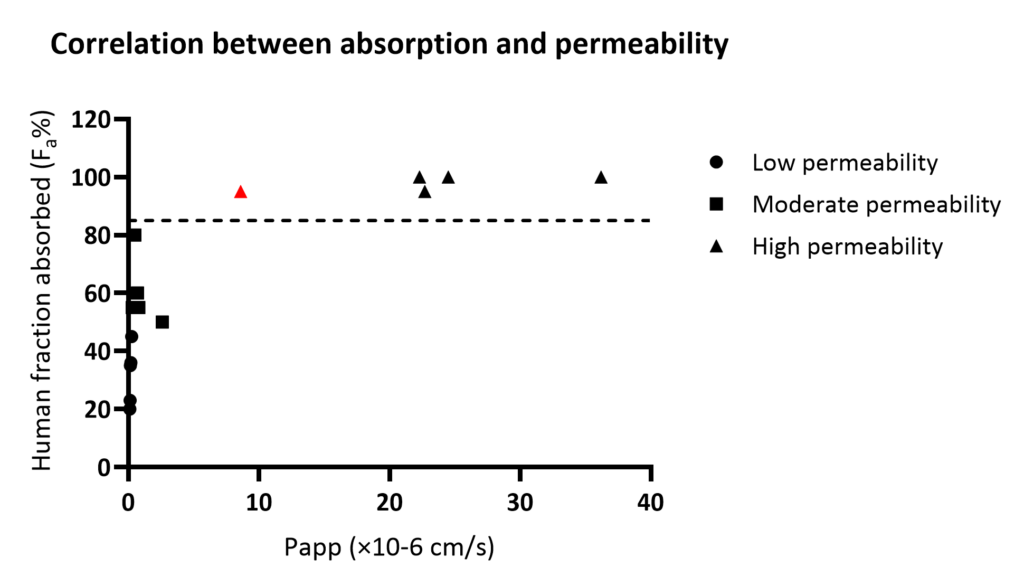
Our ICH M9-compliant in vitro permeability assay has been validated with 15 reference compounds in Caco-2 cells. The figure below illustrates the relationship between calculated Papp (A to B) and human oral absorption data (Fa), with a high permeability threshold (Fa ≥ 85%) marked as a dotted vertical line. A high-permeability internal standard is highlighted in red.
The assay evaluates first passive transport with appropriate controls, while the following bidirectional assay allows the permeability classification. Naturally, the protocol contains necessary permeability control compounds, as well as a control for Pgp efflux. TEER measurements ensure the integrity of the monolayer before and after the experiment, and LC/MS/MS quantifies intracellular concentrations for mass balance permeability determination, when necessary.
By providing accurate permeation coefficients (Papp), our assay classifies compounds as high or low permeability, delivering a comprehensive report to support regulatory submissions and drug development. We also offer solubility determination and in vitro dissolution studies to further support your project.
Reach out to our team to discuss more or ask for a quote through our Admeshop!

New E-Book Release
We are excited to announce the continuation of our e-book series with Preclinical Characterization of Biologics: Part 2.
This e-book focuses on two types of biological drugs: antibody-drug conjugates (ADCs) and oligonucleotides. Both modalities offer unique advantages and present distinct challenges in drug discovery and development. The e-book provides a comprehensive overview of their pharmacokinetics, preclinical ADME characterization, and the analytical techniques commonly used to study them.
Don’t miss this interesting read, download your free copy now from our website!
