Understanding transporter-mediated drug disposition is an integral part of preclinical and clinical drug development. Localized in cell membranes, drug transporters facilitate the movement of drugs in and out from cells. Drug transporters are categorized into two main superfamilies: the ABC (ATP-binding cassette) and SLC (solute carrier) transporters. In principle, the ABC transporters are efflux transporters that pump drugs out from cells while SLC transporters mainly function as cellular uptake transporters. Due to the wide expression of drug transporters in pharmacokinetically important tissues, these proteins can determine the absorption, distribution, and elimination of drugs. For example, intestinal ABC transporters P-gp (MDR1) and BCRP can limit oral absorption of drugs by effluxing them back to the intestinal lumen. Similarly, in the blood-brain barrier (BBB), these ABC transporters can limit the drug’s distribution to the central nervous system. On the other hand, hepatic SLC transporters (such as OATP1B1 and OATP1B3) can determine uptake of drugs into hepatocytes whereas hepatic ABC transporters pump drugs back to the blood, or into bile. In the kidney, renal SLC transporters (such as OCT2, OAT1, OAT3) and efflux transporters (MATE1, MATE2-K, P-gp) can mediate the renal excretion of drugs.
In principle, there are two types of in vitro transporter studies: identification of the major transporters contributing to active drug transport (substrate studies) and evaluation of the drug as an inhibitor of transporters (inhibition studies). In drug discovery or early clinical development, screening for transporter substrates or inhibitors is used to support selection of drug candidates with ideal ADME-Tox properties. For example, bypassing P-gp and BCRP-mediated efflux can be an efficient strategy to increase oral bioavailability or brain penetration of CNS targeted drugs. Similarly, mitigating inhibition of bile acid transporters, such as the bile salt export pump (BSEP), is preferred to minimize the risk for drug‐induced liver injury (DILI). In addition to optimizing and understanding drug disposition and toxicity, drug transporter studies are important in the investigation of drug-drug interactions (DDI) before initiating clinical studies in patients.
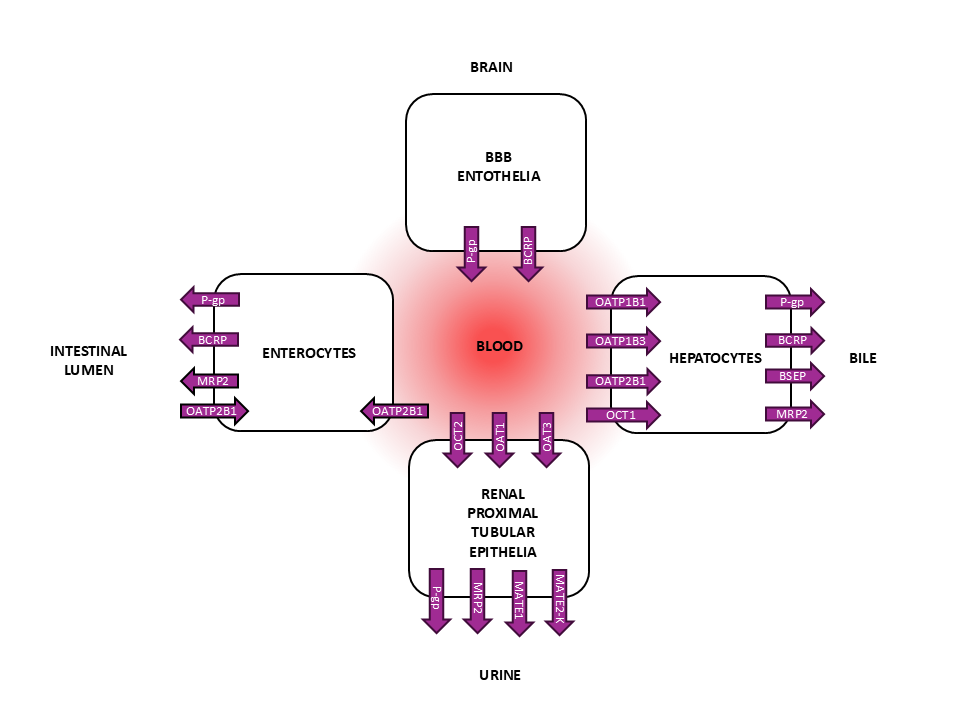
The International Council for Harmonization (ICH) guideline for drug interaction studies (ICH Harmonized guideline – Drug interaction studies M12) describes requirements for the in vitro (and clinical) transporter studies. These regulatory studies are used to evaluate if the investigational drug has the potential to be an object/substrate (affected by other drugs) or precipitant/inhibitor (the investigational drug affects the pharmacokinetics of other drugs) of drug transporter-mediated DDIs. Thus, in vitro transporter substrate and inhibitor studies are an integral part of regulatory preclinical studies.
Although around 500 genes encode for the two major transporter superfamilies, the ABC and SLC transporters, the clinical evidence is established currently only for a handful of transporters to be routinely studied in DDIs. For the regulatory transporter substrate studies, information on the intended route of administration, absorption, elimination pathway, site of action, and passive permeability of the drug candidate can be used to select the relevant transporters. In principle, due to the broad expression (e.g. intestine, liver, brain, kidney) and clinical significance of P-gp and BCRP, most drugs are evaluated for being substrates of these ABC efflux transporters. The primary SLC transporters to be assessed are the hepatic OATP1B1, OATP1B3 and renal transporters OAT1, OAT3, OCT2, MATE1 and MATE2-K. According to the ICH (M12) guideline, the hepatic transporters should be studied when hepatic metabolism or biliary excretion are major elimination pathways (≥25% of elimination) or if drug’s pharmacological target is in the liver. Similarly, the renal transporters should be evaluated if renal active secretion is ≥25% of the drug’s systemic clearance. In some cases, however, a more comprehensive transporter panel is needed to understand or predict preclinical or clinical results. For example, if the aforementioned transporters and passive permeability cannot explain the absorption, elimination or DDI of the investigational drug, additional transporters, such as OATP2B1, MRPs, and OCT1, should be evaluated.
Regarding regulatory transporter inhibition studies, the ICH (M12) Guideline states that at least the 9 relevant transporters (P-gp, BCRP, OATP1B1, OATP1B3, OAT1, OAT3, OCT2, MATE1, MATE2-K) should be evaluated regardless of the elimination route. Even if hepatic or renal elimination would not be a relevant pathway, the investigational drug can still inhibit transporters at these sites and affect the pharmacokinetics of other drugs. Furthermore, inhibition of other transporters should be assessed if the drug is commonly co-administered with known substrates of other transporters.
In general, three types of in vitro systems are applied to study the transport of a compound: 1) uptake of compound into transporter-expressing recombinant cells or membrane vesicles, 2) permeation through polarized (recombinant) cell monolayers, 3) and whole-cell systems such as primary cells. With increasing system complexity and in vivo resemblance, the system’s capability to differentiate between individual transporters tends to decrease. Therefore, the choice of the most suitable method should reflect the study question. For example, if the aim is to assess interactions of a specific transporter, the recombinant cell systems are ideal to minimize confounding factors on result interpretation. On the other hand, if the goal were to have an overall estimation of the contribution of passive and active uptake processes and the interplay of transporters and/or enzymes, whole-cell systems are more suitable.
For the assessment of interactions with a specific SLC transporter, recombinant cells over expressing a single transporter are the preferred method. The HEK293 cell line is a widely applied system for its human origin and low background endogenous transporter expression. SLC-transporter substrate studies can be conducted in these cells by comparing the uptake of the investigational drug into transporter overexpressing cells relative to parental or mock transfected control cells. A higher uptake of the drug in transfected cells that is reduced in the presence of a known transporter inhibitor is regarded as an indicator of drug being a substrate for the specific transporter. In transporter inhibition studies, the uptake of a known transporter probe substrate is monitored in the presence of the investigational drug. Transporter inhibition is observed as decreased cellular accumulation of the probe substrate in the presence of the drug.

To study specific ABC efflux transporters, an uptake transport mechanism (active or passive) into cells is required for the efflux transporters to be able to interact with the investigational drug. Therefore, efflux transporters are commonly studied in inside-out membrane vesicles isolated from transporter-expressing recombinant cells. In these vesicles, the drug has direct access to the transporter in the membrane. In inhibition studies, the ATP-dependent transport of a known efflux transporter substrate is monitored in the presence and absence of the investigational drug. In principle, the vesicle method can be applied for inhibition studies of most drugs. However, the utility of the vesicle system in efflux transporter substrate studies is limited for medium/high permeability compounds that may have high nonspecific binding to the vesicle membrane and/or extensive diffusion back from the vesicular compartment. Therefore, knowledge on permeability of the investigational drug is used to guide the selection of the appropriate system for ABC transporter substrate studies. For medium/high permeability compounds, monitoring the flux of the drug through polarized cell monolayers (such as MDCK) are advantageous. These cells can be transfected to overexpress a single ABC transporter (such as P-gp or BCRP) to study interactions mediated by a transporter. However, the cell monolayer assays are not suitable for low permeability drugs that cannot cross the cell membranes and, consequently, the membrane vesicle system is an alternative for such compounds.
Aiming for a more holistic understanding of drug transport, freshly isolated or cryopreserved hepatocytes or the Caco-2 cell line are widely used to predict hepatic and intestinal permeation. For example, suspension or plated hepatocytes are helpful in evaluating the roles of passive and active hepatic uptake mechanisms, determining hepatic and biliary clearance rates, and identifying compounds transported by hepatic transporters. Similarly, because the Caco-2 cells have resemblance to human intestinal enterocytes, this model is used to predict oral intestinal absorption of drugs. However, as fully selective transporter substrates and inhibitors are not available, the interpretation of results from these models can be complicated.
Feel free to reach out to our experts to discuss the most suitable drug transporter assessment strategy for your project!

