Building on our ongoing R&D advancements, we’re excited to announce the expansion of our in vitro toxicology portfolio with the launch of our new service package, DILIscope!

What’s Included in DILIscope?
Drug-induced liver injury, DILI, often leading to acute liver failure, can be caused by several mechanisms and poses a challenge in drug discovery. Our approach to assess and mitigate the risk for hepatotoxicity and DILI utilizes automated high-content imaging including evaluation of early signs of hepatotoxicity and cell stress, steatosis, phospholipidosis and lysosomal trapping. Importantly, the assays employ pooled primary human hepatocytes to achieve complete metabolic function and acknowledge inter-individual variability in humans. With all these parameters evaluated, one can confidently screen for potential DILI risk related to the compounds and identify hazards. Furthermore, to gain further complimentary information, the compounds can be tested for whether they form reactive metabolites or inhibit transporters, which are also mechanisms of liver toxicity.
Hepatotoxicity and cell stress
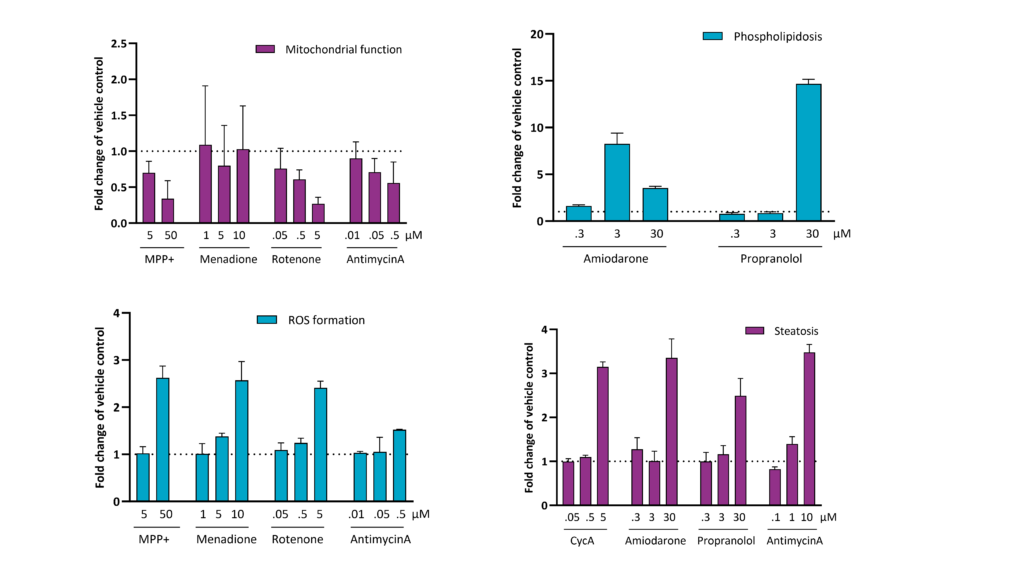
The sensitive high-content screening cytotoxicity assay, conducted in pooled human hepatocytes, delivers multiple endpoints elucidating the mechanism behind potential hepatotoxicity. The assay gives information about basic functions such as cell loss and viability, membrane integrity as well as nuclei size and variation in their intensity. Additionally, mitochondrial function and production of reactive oxidative species are included; these are often the first signs of cellular toxicity and typical mechanism inducing DILI.
Steatosis and phospholipidosis
Steatosis, marked by the buildup of fatty acids and lipids, and phospholipidosis, a sign of lysosomal malfunction and accumulation of phospholipids and lamellar bodies, may arise at concentrations of drugs lower than those causing cytotoxic effects, thereby increasing the risk for hepatotoxicity and pathogenic conditions such as of drug-induced fatty liver disease or steatohepatitis.
Lysosomal trapping
Certain types of compounds may be prone to lysosomal trapping or sequestration, which impacts their pharmacokinetics, efficacy, and safety properties, and is also one of the mechanisms inducing phospholipidosis. Our cutting-edge lysosomal trapping assay utilizes Lysotracker dye to evaluate the lysosomal sequestration potential of drug candidates. When the test compound undergoes lysosomal sequestration, there’s a concentration-dependent decrease in the lysosomal fluorescence signal as the compound competes with the dye, which accumulates into lysosomes.
Furthermore, BSEP, MRP2 and MRP3 inhibition and reactive metabolite screening can be included in the workflow to complement the data.
Utilizing automated high-content imaging methods, the sensitive assays are coupled with rapid analysis and reliable readouts. Reach out to our team to discuss more!
